FAQS
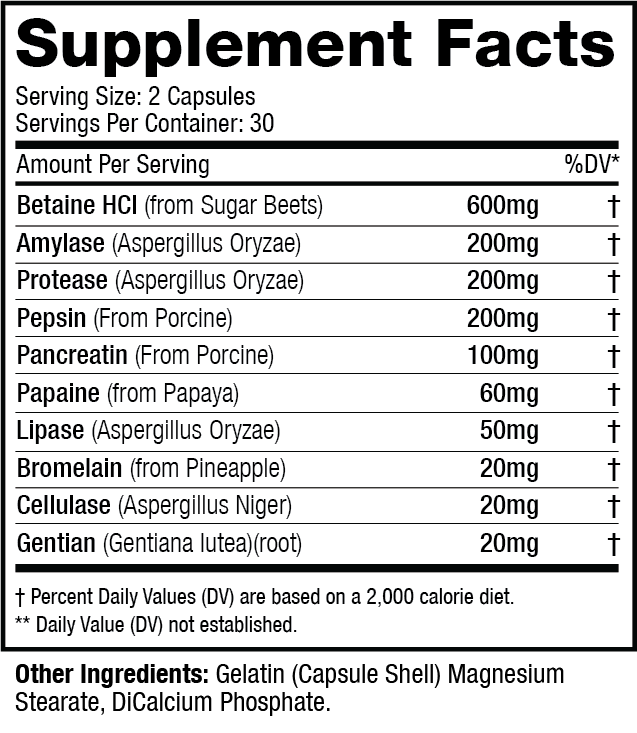
Q: What is the best way to use Digestive Enzymes Revolution?
A: As a dietary supplement, use 1 serving (2 capsules) of Digestive Enzymes Revolution prior to meals to aid the digestive process.
References
WARNING
California’s Proposition 65 entitles California consumers to special warnings.
WARNING: Cancer and Reproductive Harm - www.P65warnings.ca.gov/